
Posted by Ashley Rezak | 04/25/2024
In recent years, the landscape of pharmaceutical formulations has been witnessing a significant shift towards non-invasive delivery methods. Among these, nasal administration has emerged as a promising option, particularly in the wake of a respiratory virus pandemic and the quest for innovative drug delivery solutions. With a growing interest in nasal drug delivery, there's also a corresponding surge in products aiming to protect against respiratory infections.

Posted by Nicholas DiFranco | 04/17/2024
Oral suspensions are pharmaceutical formulations in which solid particles of active ingredients are dispersed in a liquid vehicle. These suspensions are commonly used for administering medications to patients who have difficulty swallowing solid dosage forms, such as tablets or capsules, or for those who require customized dosages. Here's a comprehensive guide to understanding and formulating oral suspensions:

Posted by Isabel Gómez | 03/28/2024
In the relentless pursuit of an active and healthy lifestyle, nutrition plays a pivotal role. Often overlooked but crucial, magnesium is an essential mineral that influences numerous biochemical reactions within the body. Addressing the need for a concentrated and effective magnesium source, MAGSHAPE™ microcapsules has emerged as a game-changer. This blog aims to delve into the nutritional significance of magnesium, the unique benefits offered by MAGSHAPE™ microcapsules, and its versatile applications for individuals aiming to maintain optimal health and well-being, including its impact on sleep quality.

Posted by Isabel Gómez | 02/02/2024
In 2024, our goal is to provide Lubrizol solutions that align with consumer trends. We will accomplish this by utilizing our branded ingredients, supported by science and technological innovation, to enable differentiated nutraceutical products. This post discusses the increasing popularity of nutraceutical ingredients and the needs of consumers that need to be addressed. It also covers popular categories that are gaining momentum, such as products that support sleep, immune health, beauty from within, and gut health.
Posted by Ashley Rezak | 01/18/2024
Topical drug delivery has long been a significant route of treatment for various ailments. Ranging from simple salves used by ancient civilizations to the sophisticated formulations of today, the progress in this sector represents the confluence of biology, chemistry, and innovative technology. But the role of a drug isn't just about its therapeutic effect. The medium through which it's delivered – the excipients – play an indispensable role in dictating the efficacy, stability, and overall performance of a topical product.

Posted by Nicholas DiFranco | 08/30/2023
Extended-release tablets, also known as sustained-release, controlled-release, or time-release tablets, have become increasingly important in the healthcare sector due to their ability to maintain consistent drug levels in the body over a specific period. This drug delivery system provides various benefits, including enhanced patient compliance, minimized side effects, and improved therapeutic efficacy.
Posted by Ashley Rezak | 08/23/2023
While advancements in drug delivery are continually improving drug efficacy and bioavailability, there are still significant areas of unmet need in the market. This post discusses some key challenges of oral drug formulations and why they are important to address for patient health and differentiation in a crowded market. We uncover strategies for overcoming these challenges, including extended drug release, taste masking, and tablet size reduction.

Posted by Ashley Rezak | 08/01/2023
Topical drug delivery is a desirable option for formulators seeking a non-invasive, patient-preferred route of administration.

Posted by Joey Glassco | 06/29/2023
It’s no secret that solubility and bioavailability issues continue to challenge formulators and drug developers.

Posted by LLS Health Technical Team | 03/17/2023
The FDA 505(b)(2) regulatory pathway for drug approval, which allows applicants to use existing safety data as evidence, continues to grow in popularity.

Posted by Nicholas DiFranco | 01/25/2023
For drug developers working on oral solid dosage drug projects, solubility, and therefore bioavailability, is a significant challenge. At present, poor water solubility impacts around 40% of drugs currently on the market.
Posted by LLS Health Technical Team | 05/03/2022
Apisolex™ technology provides formulators with a safe, polyamino-acid-based excipient to enhance the solubility of BCS Class II and IV APIs, including oncology agents, for parenteral delivery.

Posted by LLS Health Technical Team | 04/14/2022
Oral solid dosage forms, such as tablets, represent the largest segment of the pharmaceutical market. Despite the long history of oral solids in pharmaceuticals, there is still high demand for innovation. For new oral drugs to be commercially successful, it is key that they are easy to manufacture, cost-effective, and patient-friendly. Excipient technologies, such as Carbopol® polymers, can enable for formulator- and patient-centric benefits for these products. Learn more in this article.
Posted by LLS Health Technical Team | 03/29/2022

Posted by LLS Health Technical Team | 02/01/2022
Whether your focus is in electrophysiology, neurovascular, structural heart, vascular peripheral intervention or specialized/localized drug delivery, working with a skilled design and development team helps you achieve desired design requirements on schedule and in budget.

Posted by LLS Health Technical Team | 01/31/2022
In this post Lubrizol Life Science Health explores the regulatory requirements governing excipient safety for the United States as an example of best practice to help formulators understand how to select the most appropriate carbomer for their needs.
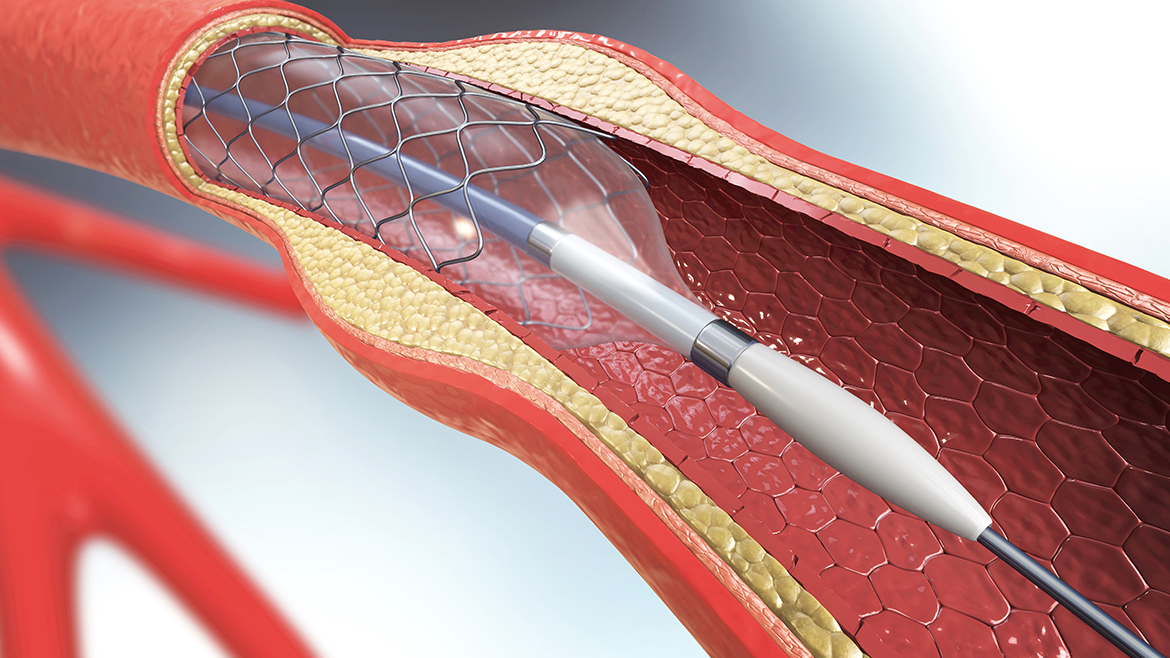
Posted by LLS Health Technical Team | 01/11/2022
Lubrizol Life Science Health is committed to providing a single-source solution that supports customers from concept to commercialization for their medical device solutions. The recent expansion of our U.S. Medical Device Design Center represents the next phase of this commitment.

Posted by LLS Health Technical Team | 11/10/2021
OEMs and startups alike increasingly see the advantages of working with an integrated medical device development provider that can support projects from concept to commercialization.
Welcome to the Blog. Our mission is to accelerate your success through partnership and proactive innovation. This blog will provide unique perspectives from the best in the business. We are dedicated to sharing our extensive market knowledge and formulation expertise to help our partners improve patient outcomes.