Growth in ocular drug development
Drug delivery to the back of the eye, known as the posterior segment, has been the focus of the ophthalmic industry in recent years. Several breakthrough therapies have emerged in this space, such as VEGF inhibitors and biodegradable intravitreal implants, as newfound innovations are allowing formulators to more effectively target the posterior segment. There is no doubt that complex injectables such as these play an important role in the pharmaceutical market, especially when it comes to outsourced services and CDMOs.
Despite the recent growth in ocular injectable products, topicals remain the most prominent dosage form for ophthalmic commercialized products and those in development. This trend has resulted in an all-encompassing surge in the development of treatments for eye disease. However, all ophthalmic dosage forms still face a variety of formulation challenges. We sat down with Dr. Barbara Morgan, Global Pharmaceutical Business Director at Lubrizol Life Science Health (LLS Health), to explore industry trends and methods for overcoming obstacles imposed by the anatomy and chemical make-up of the eye.

Figure 1: Quote on Topical Ophthalmics
What is anterior ocular drug delivery and what are the challenges associated with it?
Ocular drug delivery can be grouped into two categories, posterior and anterior drug delivery, each with its own distinct obstacles. Anterior drug delivery affects the front portion of the eye and generally deals with penetrating the cornea. The cornea is the transparent layer covering the visible portion of the eye and serves to focus light. It is most commonly targeted with topical liquid formulations such as eye drops, including solutions, suspensions, and emulsions.
“The issue with topicals is that, typically, only 1-5% of the administered drug in a standard eye drop is absorbed at the site of action,” notes Barbara. “This is primarily due to the tricky path a drug must take to effectively penetrate the target tissue.”
Some of the main hindrances in topical ocular delivery result from the inherent nature of standard topicals, as well as the natural mechanisms of the eye. The average eye drop is 30-50 μL, whereas the tear film capacity of the eye is only 6-8 μL, resulting in a large waste of product and presenting a significant delivery limitation.
For the little liquid that does remain, innate solution drainage and blinking make it difficult to keep a topical drug product in place long enough to be absorbed. Without incorporating differentiated ingredients or technologies that help overcoming these challenges, frequent administration of the drug product is required to maintain an effective drug level. However, this can lead to:
- Toxicity and overdosing
- Decreased patient compliance due to added inconvenience
- Increased cost if dealing with an expensive active pharmaceutical ingredient (API).
How can formulators overcome challenges in anterior ocular drug delivery?
“Well, there is no one magic answer,” says Barbara, “but, in addition to making the drug more soluble for absorption by the cornea, which is naturally very impervious, the best way to enhance drug delivery to the anterior segment is by prolonging a drug’s residence time on the surface of the eye. For eye drops, the key there is muco- or bioadhesion.”
Bioadhesion is commonly defined as the adherence between two surfaces, at least one of which is biological. In the case of topical ophthalmic delivery, the surface of the liquid drug product and the front of the eye are the relevant interfaces. Bioadhesion can be introduced in a formulation via a variety of ingredients and delivery systems.
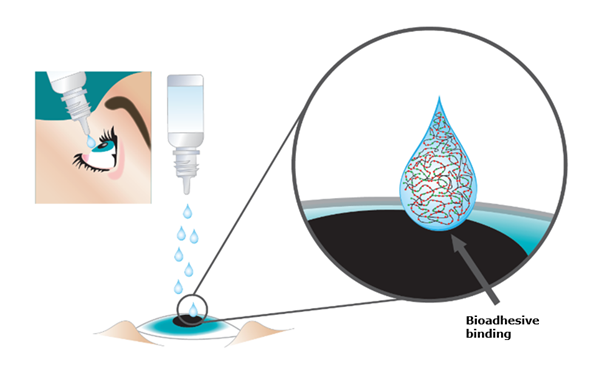
Figure 2: Demonstration of Bioadhesive Binding
“There are some drug product developers working on advanced delivery systems to instill bioadhesive properties, such as those utilizing solid lipid nanoparticles, micelles, and liposomes. Some of these are very promising and it’s exciting to see so much innovation in the ophthalmic space,” states Barbara. “However, I would have to say one of the most effective and well-established methods to impart muco- or bioadhesion to a formulation is through strategic excipient selection.”
There are many polymers a formulator could select for establishing bioadhesion in a formulation, such as carbomers, carboxymethylcellulose, polycarbophil, or sodium alginate, to name a few. LLS Health is a consumer-driven partner offering several multifunctional bioadhesive excipients, such as Carbopol® polymers and Noveon® polycarbophil.
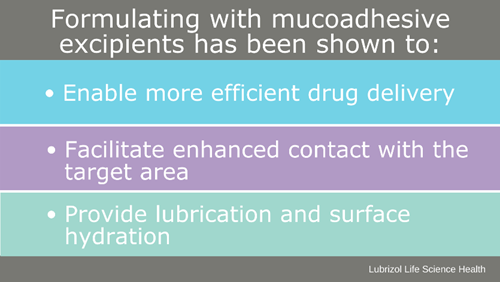
Figure 3: Benefits of Bioadhesive Excipients
What are common obstacles encountered with back-of-the-eye drug delivery and how can you overcome them?
“For posterior ocular diseases, topicals and bioadhesive excipients are not enough to facilitate effective drug delivery,” notes Barbara. “The back of the eye is regarded as one of the most difficult areas to effectively treat. Compared to the anterior portion, the posterior segment generally cannot be accessed via a topical route due to the natural impermeability of the eye exterior and the distance a drug would have to travel to reach the site of action, which is usually the retina.”
The retina is comprised of a thin layer of tissue lining the back of the eye. This tissue receives light, which it then converts into neural signals and sends to the brain for recognition. Unsurprisingly, disorders affecting this area are one of the leading causes of vision loss, making the retina a primary focus of many posterior treatments.
Since topicals and systemic treatments via intravenous drug delivery, due to toxicity issues, are typically not practical methods of treating the retina, this leaves ocular injections and implants as the primary methods for overcoming back-of-the-eye barriers and delivering effective therapies.
How are ocular challenges shaping industry trends?
“Due to the surge in interest on ocular parenterals for retinal disorders, as well as the continued focus on ocular topicals for anterior therapies, we are seeing some recurring themes with new products on the market and in development,” remarks Barbara. These include:
- Growing concentration on non-preserved formulations, including sterile multidose ophthalmic bottles
- Development of combination therapies
- Increasingly complex formulations, such as sustained-release systems
- Focus on therapies for retinal disorders
- Activity preceding the impending generics boom, as several critical drugs are about to come off patent
“These trends are already evolving the ophthalmic pharmaceutical space, which we can see firsthand with the significant uptick in venture capital funding to the ocular segment, introduction of preservative-free products like the RESTASIS Multidose®, and increased projects focusing on ocular injectables and implantables, whether for a generic, 505(b)(2), or new active,” says Barbara.
Sterile multidose bottles
While ophthalmic bottle formulations are typically prepared with preservatives to prevent contamination, there is a recent push to move away from these substances due to toxicity and irritation concerns. Developers have begun to look for alternatives to common preservatives like benzalkonium chloride (BAK) and single-use packaging, which can be preservative-free but often expensive. This has given rise to the use of sterile multidose bottles, such as the one we have seen with the RESTASIS Multidose® bottle produced by Aptar Pharma. Other multidose bottle manufactures include Aero Pump GmbH and Nemera, with several other players looking to enter this space due to the recent growth.
Combination therapies
For disorders like glaucoma or after a surgery such as that for cataracts, multiple different drugs are often needed for effective treatment. These often consist of anti-inflammatories and topical analgesics, among others, and are typically administered frequently as various eye drops. However, this can be a hassle for patients and often leads to poor compliance due to the frequency of therapeutic administration. As a result, pharma companies are exploring API-combination topicals, which allow for only one set of eye drops instead of multiple bottles needing to be administered at different times.
Sustained-release systems & retinal therapies
Since many patients affected by retinal disorders are over 50 and standard treatment methods can be highly invasive, patient compliance can be a significant issue with posterior ocular disorders. Sustained release therapies have the advantage of efficacy lasting several weeks to months, or even years, which reduces frequency of treatment administration and subsequent consumer usage. The pharmaceutical industry has recognized this unmet need and has launched several long-acting ophthalmic injectables and implants. Ocular implants make up approximately one-third of all FDA-approved implants on the market and have almost double the number in clinical development. Sustained release is also critical in ocular topicals and seen most commonly in the form of bioadhesion to the cornea or conjunctiva for prolonged drug contact time with the eye.
Generics boom
With the first generic of blockbuster dry eye drug RESTASIS® estimated to launch any day now, and age-related macular degeneration (AMD) injectables Lucentis® and Eylea® coming off-patent this year, the ophthalmic market is already buzzing with generic product development. Several other ophthalmic drug products will hit patent expiration in the next few years and we will surely see a surge in FDA abbreviated new drug application (ANDA) approvals.
Despite the challenges, do you see growth in the ocular market?
“Absolutely! Through our industry colleagues and customers, we’ve witnessed a ton of advancement in the ophthalmic space, with more to come. Posterior disorders are getting a lot of attention, but I’m particularly interested to see what arises with ocular topicals for front-of-the-eye diseases,” notes Barbara. “Between 2015 and 2018 an 800% increase was observed in novel ocular drug approvals, a portion of which were topical. Dry eye disease and glaucoma are two of the top ophthalmic indications with treatments in development, and the primary therapy there is eye drops.”
There is still significant unmet need in all ocular drug product formats and the industry is primed for an onset of growth. Ophthalmic health is becoming increasingly significant, as we continually see a rise in digital screen time, population age, and airborne pollution. The World Health Organization estimates that around 2.2 billion people around the globe experience some form of vision impairment.
LLS Health is powered by the partnerships we forge with our customers and driven by consumer needs, with the ultimate goal of creating solutions that improve patient lives. We are enabling formulators to innovate and meet this growing ocular demand through integrated excipient functionality, CDMO services, and continuous technical and regulatory support. At LLS Health, the difference is inside – contact us today to learn more.
Authors:
Barbara Morgan | Global Pharmaceutical Business Director, LLS Health
Ashley M. Rein | Technical Marketing Specialist


