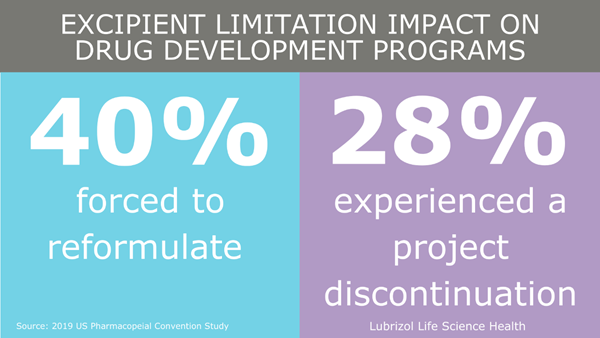
Excipient (inactive ingredient) selection and qualification for use in a drug product is critical to the success of any pharmaceutical development program. Pharmaceutical excipients are substances other than the active ingredient that have been evaluated for safety and are intentionally included in a drug delivery system. Excipients are vital for ensuring essential drug product properties, such as increasing bioavailability or enabling controlled release of a drug.
However, the current regulatory landscape, or lack of an independent excipient approval pathway, may be limiting the entrance of new excipients in the market and, subsequently, new drug products. We sat down with the Director of Regulatory Strategy and Policy for Lubrizol Life Science Health (LLS Health), Meera Raghuram, to discuss the importance of novel excipients and recent actions taken by the FDA.
Q: What is a novel excipient, and how does an excipient become approved?
“Well, for starters, it’s actually a common misconception that excipients are ‘approved,’” says Meera. “Inactive ingredients themselves are never actually approved by the FDA. Excipients are reviewed as a component of the finished drug product when a New Drug (NDA), Abbreviated New Drug (ANDA) or a 505(b)(2) application is submitted. This sets the precedence of use for the excipient in an approved drug product and therefore the excipient is no longer considered ‘novel’ in that particular route of administration at the approved maximum daily intake levels.”
A novel excipient generally refers to an inactive ingredient that has not been previously used in an approved drug product in the United States. However, regulators may view an excipient that has not been used in a particular route of administration or at levels above the precedence in an approved drug product as “novel.” Even if an excipient has been extensively used in other FDA-approved, non-pharmaceutical applications, such as food or OTC products, it is still considered “novel” when used in drug products subject to regulatory approval. In general, drug manufacturers are reluctant to use a novel excipient in a drug product as there is no certainty that FDA would find the available safety information on an excipient adequate. This uncertainty is greater for generic drugs as non-clinical and clinical studies are not required for regulatory approvals.
“Since there is currently no pathway for excipients to be evaluated independently, it is only once an excipient is present in an approved drug product that it is no longer considered novel and will appear on the FDA Inactive Ingredients Database (IID),” Meera notes.
Q: What is Inactive Ingredients Database (IID)?
The IID is a key repository of excipient information that a drug developer can use to evaluate potential inactive ingredients for their formulation. This database provides information on the maximum potency per unit dose of excipient in approved drug products in the United States for a particular route of administration.
“Once an excipient is listed in the database, it will require less extensive FDA review the next time it is included in a similar type of drug product since precedence of use and safety for a particular route of administration would have been established,” comments Meera. “Understandably, this often sways developers towards only using IID-listed ingredients even if not all optimal performance aspects for the drug may be met.”
Q: Is only using IID-listed excipients an issue?
Q: What is the FDA doing to remedy the situation, and how is the industry playing a role?
“Based on discussions with various stakeholders and their expressed concerns, the FDA is considering developing a pilot program for the toxicological and quality evaluation of novel excipients and has sought stakeholder input. The program would be voluntary and would allow for review of a limited number of submissions per year. Excipient suppliers and users, including LLS Health and industry associations alike, have submitted comments in response to the FDA proposed program with majority in support of the program. The overwhelming support seems to prove the definitive need for a novel excipient program like this.”
Q: What are the potential benefits of a Novel Excipient Review Program?
“A program like this could unlock a wealth of previously untapped potential when it comes to new products and treatment options. Regulatory recognition of these important new excipients early in development will encourage more widespread consideration of their application, creating more versatility in drug delivery and manufacturing method” states Meera.
As summarized in the comments submitted to the FDA, potential benefits for future drug products as a result of the Novel Excipient Review Program may include:
- Improved product performance in terms of stability, solubility, delivery, etc.
- Reduced overall development costs, which can ensure faster and more affordable patient access to new therapies
- Potential to extend drug release for longer timeframes or for more targeted drug delivery, resulting in improved patient compliance
- Increased formulation options with combination products or when repurposing drugs for a new dosage form or route of administration
- New capabilities for abuse deterrence in drugs prone to patient misuse, such as with opioids
Q: What is LLS Health doing in the novel excipient space?
“LLS Health offers a variety of excipients across a multitude of dosage forms, many of which are listed in the IID and have precedence of use in approved drug products. We are committed to partnering with our customers in bringing the best treatment options to market. Ensuring they are equipped with appropriate inactive ingredient options is an essential part of that, which is why we support the creation of this new FDA program,” Meera notes.
Many LLS Health excipients have not yet been listed on the IID but have the potential to provide significant benefits for formulations nonetheless, some of which include:
- Enhanced adhesion to mucous or biological membranes (muco- or bioadhesion)
- Better controlled drug release and improved penetration into the target tissue
- Greater versatility in processing, such as with polymers that allow for easy dispersion in water or that can be directly compressed into tablets (Carbopol® Ultrez 10 and 71G NF)
- Improved rheology control and viscosity modification with optimal aesthetics and feel
Authors:
Meera Raghuram | Director, Regulatory Strategy and Policy
Ashley M. Rein | Technical Marketing Specialist
References: